Research focus
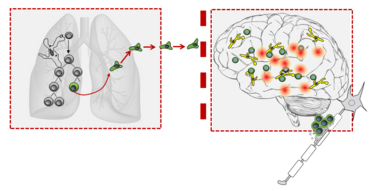
The general understanding is that there are several essential steps to developing a CNS immune disease. The disease is caused by T cells that target the CNS. Such T cells are also found in the immune repertoire of healthy people. It is only when the T cells are activated in peripheral organs that they are able to develop their full disease-causing potential: such an activation allows the T cells to pass through the blood-brain barrier, a special protective “wall” in the brain, and on into the brain tissue. Once there, the T cells effect a strong inflammatory reaction with subsequent damage to the nervous tissue (Fig. 1).
This sequence of steps, which have been consistently come to light during the last decades of multiple sclerosis research, emphasize that autoimmunity of the CNS cannot be seen as an isolated conflict between the immune system and the nervous system. On the contrary, it is a systemic process that encompasses different tissues of the body. Certain events have to take place in all these different tissues before processes leading to the actual CNS damage can occur.
My research group aims at identifying which of a sequence of different events are the decisive checkpoints that allow T cells to attack the CNS.
Specifically, we seek to answer the following questions:
- Where and how are T cells activated in the peripheral organs?
- How do T cells behave at the CNS border?
- What mechanisms are responsible for damage in grey or white matter?
Key technologies
In my research group we combine modern microscopy techniques with morphological and functional analyses to examine the behavior and function of CNS-reactivated T cells. Here, we employ a murine model of multiple sclerosis, namely experimental autoimmune encephalomyelitis.
In principle, T cells differ from most other cell types in the adult organism in that they possess the unique ability to move freely and to migrate between different organs and regions of the body. In the healthy state this ability allows these immune cells to carry out their protective function, but in a diseased state it allows them to cause damage to almost all organs of our body, for example in the case of CNS autoimmunity. Therefore, to understand (auto)immunity one has to consider the fundamental dynamic processes governing immune cells..
Over recent years the Odoardi research group has specialized in recording in living tissue the complex choreography of T-cell migration and its function using 2-photon microscopy. For a deeper morphological analysis, we employ confocal and STED microscopy. Functional characterization of the cells is achieved via a number of different techniques: mRNA analysis with quantitate PCR and transcriptome sequencing, and flow cytometric protein expression analysis.
Projects
The role of the lung in CNS autoimmunity
A central question in neuroimmunology is where and how resting CNS-reactive T cells in the periphery are activated. The lung, as we discovered in our previous work, plays an important role here (Odoardi et al. 2012). The lung is in constant communication with the world external to the body and is equipped with its own specialized immune system and microbiome. We found out that before CNS-reactive T cells invade the CNS, they first head for the lung, where they undergo a wide-ranging modification (Fig. 2). They change their characteristic motility pattern and gene expression profile in such a way to license them to breach the blood-brain barrier and transmigrate into the nervous tissue. Currently we are investigating whether the lung can also act as a trigger for CNS autoimmunity and whether the local lung milieu influences this process.
The behavior of immune cells at the border to the CNS
The CNS is surrounded by multi-layered lining called the meninges, consisting of three thin layers of tissue: the dura mater, the arachnoid membrane and the pia mater. They provide an outer shield for the CNS (Fig. 3).
The meninges play an important role in experimental autoimmune encephalomyelitis, among other thing in initiating the autoimmune process. We could show that the meninges serve as the entry port for T cells into the CNS and they are an important checkpoint for local T-cell activation (Bartholomäus et al. 2009, Schläger et al. 2016). Currently we are researching the physical, cellular and molecular mechanisms that enable T cells to adhere to the meningeal blood vessels and to enter into the CNS tissue. We also seek to understand whether and, if so, how a meningeal inflammation can lead to nervous tissue damage.
Mechanisms of cell damage
In multiple sclerosis there are focal, demyelinating lesions in the white matter. However, the grey matter is also affected, even in the early stages. It seems that grey matter damage plays a significantly greater role in the occurrence of functional and cognitive impairments. The mechanisms responsible for this damage to the grey and white matter are not yet sufficiently understood. Recently, we could show in an experimental animal model that autoreactive T cells directed against neuronal structures initiate a strong inflammation in the grey matter (Lodygin et al. 2019). This model is particularly important because most common animal models for MS only cause damage to the white matter (Fig. 4). Currently we are researching what exact mechanisms play a role in damage to the grey or white matter.
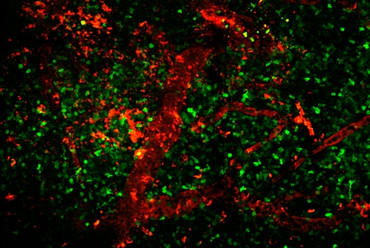
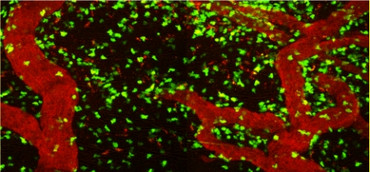
Fig. 4: Spatial distribution of T cells directed against neurons (TbSYN) or myelin (TMBP) in the brain or spinal cord, respectively. 2-photon fluorescent microscopy.
Contact

contact information
- telephone: +49 551 3961139
- e-mail address: francesca.odoardi(at)med.uni-goettingen.de
Relevant Publications
Hosang L, Löhndorf A, Dohle W, Rosche A, Marry S, Diercks B-P, Müller-Kirschbaum LC, Flügel LT, Potter BVL, Odoardi F, Guse AH*, Flügel A*.
2-Methoxyestradiol-3,17-O,O-bis-sulfamate inhibits store-operated Ca2+ entry in T lymphocytes and prevents experimental autoimmune encephalomyelitis.
Biochim Biophys Acta Mol Cell Res (2023), 1870: 119485. doi: 10.1016/j.bbamcr.2023.119485. *equal contribution. PMID 37150482.
Merlini A, Haberl M, Strauß J, Hildebrand L, Genc N, Franz J, Chilov D, Alitalo K, Flügel-Koch C, Stadelmann C, Flügel A*, Odoardi F*
Distinct roles of the meningeal layers in CNS autoimmunity.
Nat Neurosci (2022), 25: 887-899. doi: 10.1038/s41593-022-01108-3. *equal contribution. PMID 35773544.
Hosang L, Cugota Canals R, van der Flier FJ, Hollensteiner J, Daniel R, Flügel A* and Odoardi F*
The lung microbiome regulates brain autoimmunity.
Nature (2022), 603: 138-144. doi: 10.1038/s41586-022-04427-4. *equal contribution. PMID 35197636.
Lodygin D+, Hermann M+, Schweingruber N, Flügel-Koch C, Watanabe T, Schlosser C, Merlini A, Körner H, Chang H-F, Fischer HJ, Reichardt HM, Zagrebelsky M, Mollenhauer B, Frahm J, Stadelmann C, Kügler S, Fitzner D, Haberl M, Odoardi F* & Flügel A*+equal contribution, * co-senior authors
β-Synuclein reactive T cells induce autoimmune CNS grey matter degeneration.
Nature, 566 (2019): 503-508. PMID 30787438.
Schläger C, Körner H, Krueger M, Vidoli S, Haberl M, Mielke D, Brylla E, Issekutz T, Cabañas C, Nelson PJ, Ziemssen T, Rohde V, Bechmann I, Lodygin D, Odoardi F, Flügel A.
Effector T-cell trafficking between the leptomeninges and the cerebrospinal fluid.
Nature 530 (2016): 349-353.
Lodygin D, Odoardi F, Schläger C, Körner H, Kitz A, Nosov M, van den Brandt J, Reichardt HM, Haberl M, Flügel A.
A combination of fluorescent NFAT and H2B sensors uncovers dynamics of T cell activation in real time during CNS autoimmunity.
Nat Med 19 (2013): 784-790.
Odoardi F, Sie C, Streyl K, Ulaganathan VK, Schläger C,Lodygin D, Heckelsmiller K, Nietfeld W, Ellwart J, Klinkert WE, Lottaz C, Nosov M, Brinkmann V, Spang R, Lehrach H, Vingron M, Wekerle H, Flügel-Koch C, Flügel A.
T cells become licensed in the lung to enter the central nervous system.
Nature 488 (2012): 675-679
C. Cordiglieri, F. Odoardi, B. Zhang, M. Nebel, N. Kawakami, W.E.F. Klinkert, D. Lodygin, F. Lühder, E. Breunig, D. Schild, V. Kumar, K. Dornmair, W. Dammermann; B.V.L. Potter, A.H. Guse, A. Flügel.
NAADP-mediated Ca2+ signaling in effector T cells regulates autoimmunity of the nervous system.
Brain 133 (2010): 1930-43.
I. Bartholomäus, N. Kawakami, F. Odoardi, C. Schläger, D. Miljkovic, J.W. Ellwart, W.E. Klinkert, T.B. Issekutz, C. Flügel-Koch, H. Wekerle, A. Flügel.
Effector T cell interactions with meningeal vascular structures in nascent autoimmune CNS lesions.
Nature 462 (2009):94-8.
N. Müller, J. van den Brandt, F. Odoardi, D. Tischner, J. Herath, A. Flügel, H.M. Reichardt.
CD28 superagonistic antibodies elicit two functionally distinct waves of T cell activation.
Journal of Clinical Investigation 118(2008):1405-1416.
F. Odoardi, N. Kawakami, W.E.F. Klinkert, H. Wekerle, A. Flügel.
Blood-born soluble protein antigen intensifies T cell activation in autoimmune CNS lesions and exacerbates clinical disease.
Proceedings of the National Academy of Sciences of the United States of America 104 (2007): 18625-18630.
F. Odoardi, N. Kawakami, Z. Li, C. Cordiglieri, K. Streyl, M. Nosov, W.E.F. Klinkert, J.W. Ellwart, J. Bauer, H. Lassmann, H. Wekerle, A. Flügel.
Instant effect of soluble autoantigen on effector T cells in peripheral immune organs during immunotherapy of autoimmune encephalomyelitis.
Proceedings of the National Academy of Sciences of the United States of America 16 (2007): 920-25.
N. Kawakami, F. Odoardi, T. Ziemssen, M. Bradl, T. Ritter, O. Neuhaus, H. Lassmann, H. Wekerle, A. Flügel.
Autoimmune CD4+ T cell memory: Life long persistence of encephalitogenic T cell clones in healthy immune repertoires.
Journal of Immunology, 175 (2005):69-81.
N. Kawakami, U.V. Nägerl, F. Odoardi, T. Bonhoeffer, H. Wekerle, A. Flügel.
Live imaging of effector cell trafficking and autoantigen recognition within the unfolding autoimmune encephalomyelitis lesion.
Journal of Experimental Medicine, 201 (2005): 1805-14.
N. Kawakami, S. Lassmann, Z. Li, F. Odoardi, T. Ritter, T. Ziemssen, W.E.F. Klinkert, J.W. Ellwart, M. Bradl, K. Krivacic, H. Lassmann, R.M. Ransohoff, H-D. Volk, H. Wekerle, C. Linington, A. Flügel.
The activation status of neuroantigen-specific T cells in the target organ determines the clinical outcome of autoimmune encephalomyelitis.
Journal of Experimental Medicine 199 (2004): 185-197.
