Research focus
Autoreactive T cells play a decisive role in the pathogenesis of multiple sclerosis. Our research focus lies on the migratory behavior of T cells through the body, their reactivation in the CNS and T cell-mediated tissue damage. Moreover, our goal is to develop better animal models involving the newest genetic tools to allow us to better understand the different aspects of human autoimmune CNS disease and discover new therapeutic approaches.
Key technologies
Genetic manipulations of T cell lines by retroviruses
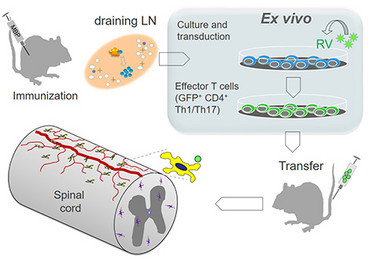
One substantial advantage of the adoptive transfer EAE model is that it provides an experimental tool to modify T cells by retroviral transduction. This procedure allows to stably or conditionally express fluorescent proteins and other genes of interest in T cells of a given specificity. We establish our effector T-cell lines by immunizing Lewis rats with a specific antigen (e.g. myelin basic protein, MBP). Then we remove the draining lymph nodes, from which we isolate MPB-specific T cells and stimulate and expand these in culture by adding antigen. At this stage the retroviral transduction takes place. These T cells are then injected into naive animals and after a prodromal period enter the CNS tissue and initiate inflammation there. T cells can be analyzed during this process by several techniques, e.g. flow cytometry, histology or multiphoton microscopy.
Transfer EAE
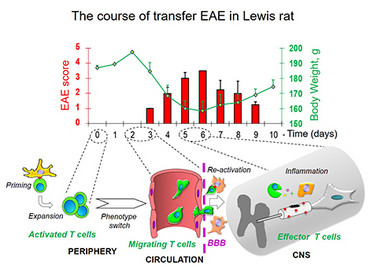
In Lewis rats, passive experimental autoimmune encephalomyelitis (EAE) can be induced by transfer of activated T cells specific for myelin antigens (e.g, MBP). The clinical course of the disease manifested by ascending paralysis is monophasic: a prodromal period is followed by a single autoimmune attack with subsequent complete recovery.
Generating transgenic rats
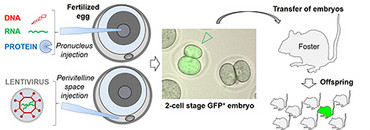
The rodent genome can be manipulated in the embryonal single-cell stage by microinjection, where nuclear acids and proteins can be injected directly into the pronucleus. Alternatively, expression constructs can be packed into lentiviral particles and injected into the embryo’s perivitelline space. After the injection double-cell stage embryos are then implanted in a pseudopregnant female animal. The resulting offspring is then tested for integration of the transgene and expression of the respective protein. Offspring which carries the desired changes can then be bred to create a transgenic line. This method can be used both to integrate a transgene (e.g. GFP-TCRtg) randomly into the genome or, with help of the CRISPR/Cas9 method, to carry out a targeted manipulation of a specific locus.
Projects
Activation of T cells in the CNS
The activation of CD4+ T lymphocytes requires assistance of antigen-presenting cells (APCs) such as dendritic cells and macrophages. APCs harvest antigens from the environment, process them to peptides and present them on the cell surface in a complex with MHC class II molecules. Binding of peptide-MHC complex to the T-cell receptor (TCR) triggers a phosphorylation cascade (e.g. Lck, Zap70, LAT) and activates phospholipase C (PLCg). This eventually leads to the release of intracellular Ca2+ from the endoplasmic reticulum (ER) and Ca2+ influx into the cytosol of T cells through CRAC channels in the plasma membrane. Raising the calcium level in the cytosol of T cells activates the phosphatase calcineurin which dephosphorylates the nuclear factor of activated T cells (NFAT) protein. This translocates NFAT to the cell nucleus, where it binds to specific promoters and activates the transcription of target genes (e.g. IL-2, IFNg, etc.), which is essential for differentiation of T cells and execution of their effector functions.
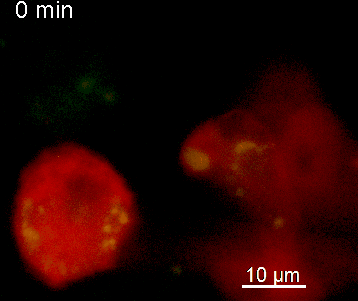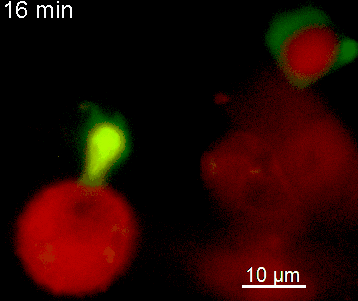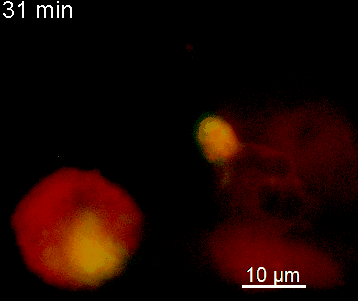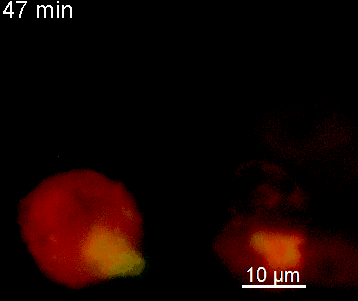
The N-terminal portion of NFAT fused to a fluorescent protein can be expressed in effector T cells by means of retroviral transduction. This enables live monitoring of the activation process using time laps epifluorescence microscopy (animation 1). The video shows dendritic cells (red), derived from bone marrow of RFP-transgenic host, presenting myelin basic protein to MBP-specific TNFAT-YFP/CherryH2B cells. The contact of a T cell with an antigen-presenting cell (APC) induces rapid nuclear translocation of NFAT into the nucleus (yellow).
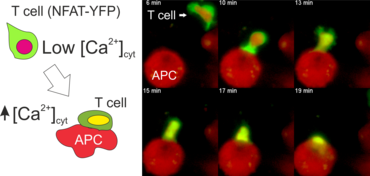
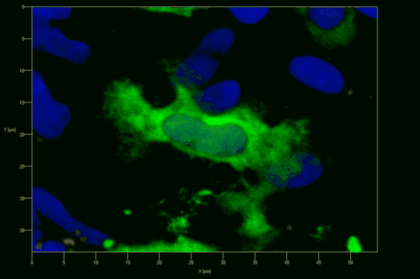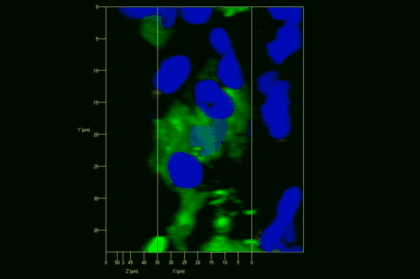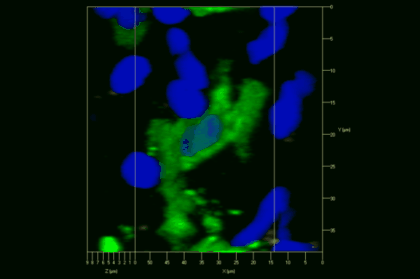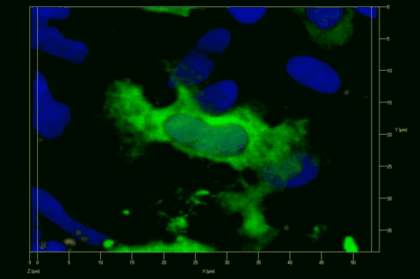
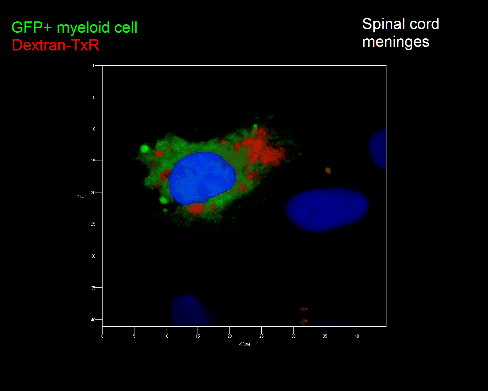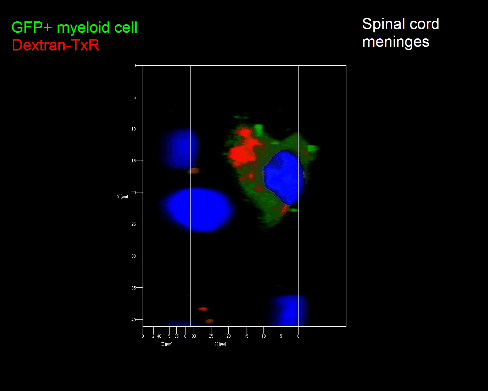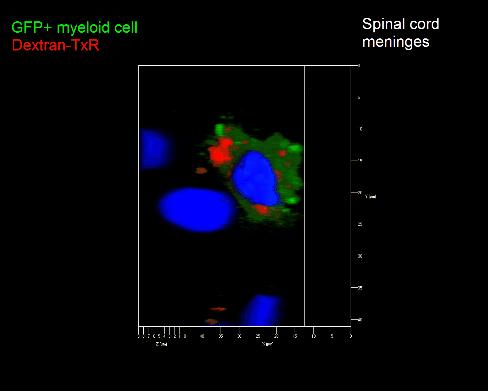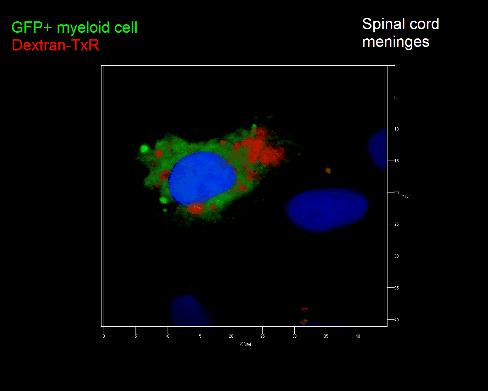
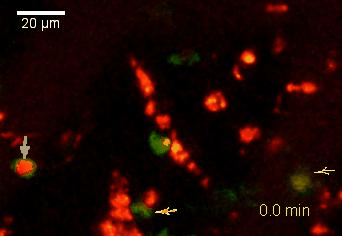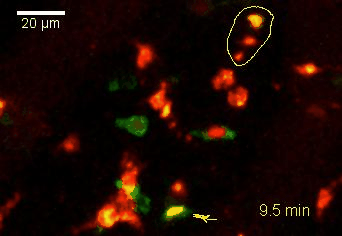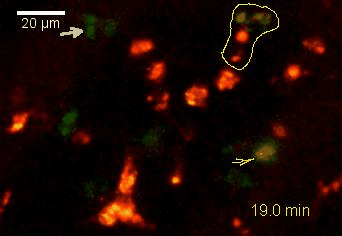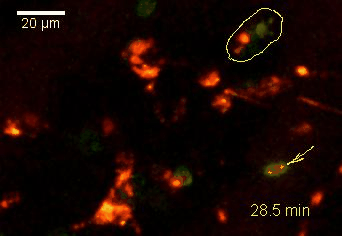
Meningeal and perivascular macrophages are the first APCs encountered by T cells crossing the blood brain barrier and entering the perivascular/meningeal space. These cells are of myeloid lineage and can be visualized in bone marrow-chimeric animals reconstituted with GFP-expressing hematopoietic stem cells (animations 2 & 3). In contrast to microglia, meningeal macrophages express a high level of MHC class II molecules on their surface. Meningeal macrophages, regarded also as phagocytes, efficiently take up macromolecules (e.g. fluorescent dextran (animations 3 and 4)) and soluble antigens. This property can be exploited to label meningeal APCs in vivo and to visualize their interaction with T cells by intravital 2-photon imaging of the spinal cord.
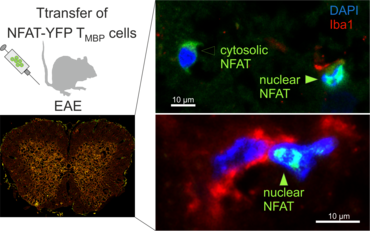
The transfer of MBP-specific T cells results in an induction of EAE disease in Lewis rats. If these T-cells express the double fluorescent NFAT/H2B reporter, they can be visualized in the meninges of the spinal cord shortly before the onset of clinical symptoms (animation 4), where they arrive from the blood circulation. Extravasated T cells crawl along pial vessels and meet meningeal phagocytes there. Upon contact of non-activated T cells (gray arrow) to dextran-TxR-labelled phagocytes, NFAT translocates to the nucleus (marked by yellow circle). This allows real time quantification of the frequency of T cell activation events in situ.
Modelling T-cell mediated autoimmunity in the transgenic rats
Antigenic receptors expressed on the surface of T cells are encoded by specialized sets of genomic segments that are assembled as functional units via V(D)J recombination during T cell development.
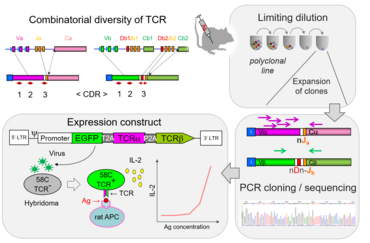
In a normal immune repertoire only few T lymphocytes are specific for a given antigen. Genetic engineering has been used to generate mice with monospecific T cell repertoires. These T cell receptor transgenic mouse strains have become extremely useful tools in immunological research. We are working on engineering transgenic Lewis rats expressing T-cell receptors (TCRs) of different specificities including autoantigens expressed in nervous tissue. To identify coding sequences for TCR alpha and beta chains, clonal population are generated using T cell culture techniques. These sequences are then integrated into lentiviral expression vectors and tested in TCR-negative hybridoma cell lines for their responsiveness to antigens. Viruses with functional TCR are used to perform transgenesis in preimplantation rat embryos to generate transgenic strains.
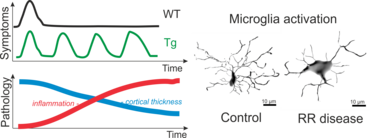
Recently, using these techniques, we were able to generate Lewis rats which express a TCR specific for rat beta-synuclein protein (Lodygin, Hermann et al., 2019). With this rat model we could show that T cells which recognize a neuronal autoantigen (b-Synuclein) trigger inflammation in the grey matter of the CNS. In contrast, myelin-specific T cells trigger inflammation in the white matter of the CNS. Additionally, we could show in our transgenic animals that repeated attacks from CD4 T-effector cells evoke permanent gliosis, neuronal damage and cortical brain atrophy. This new animal model is therefore suitable for studying mechanisms in CNS autoimmune diseases that involve progressive tissue damage.
Regulation of Ca2+ signaling in T cells
Engagement of immunoreceptors, such as TCR, results in an increase in the concentration of Ca2+ ions in the cytosol. This elevation in Ca2+ in turn activates several transcription factors including NFAT, CREB and NF-kappaB, which regulate the expression of genes essential for T‑cell function. The increase in intracellular Ca2+ levels in lymphocytes is mediated mainly through store-operated calcium entry (SOCE) and calcium-release-activated calcium channels (CRAC).
The endoplasmic reticulum (ER) and endosomes/lysosomes are intracellular stores of Ca2+. The ER in lymphocytes is relatively small, so that the release of Ca2+ from the ER to the cytosol results in a moderate and transient increase of cytoplasmic Ca2+ concentration. The concomitant depletion of the calcium stores activates CRAC channels in the plasma membrane and leads to a massive influx of extracellular Ca2+.
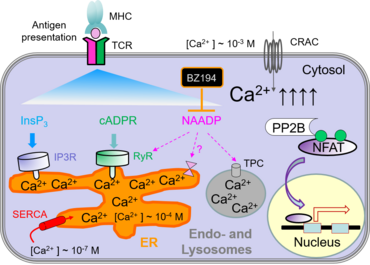
The depletion of Ca2+ stores can be triggered by several means, e.g. by second messengers, such as inositol-1,4,5-triphosphate (InsP3), cyclic ADP ribose (cADPR) and nicotinic acid adenine dinucleotide phosphate (NAADP). These second messenger molecules are generated upon activation of the T-cell receptor and signal transduction by tyrosine kinases. Recently, we have identified BZ194 as an NAADP inhibitor which interferes with Ca2+-mediated signaling during the early phase of T-cell activation (Dammermann et al., PNAS 2009). Both active and passive EAE in Lewis rats were ameliorated by preventive and therapeutic treatments with BZ194, indicating that the NAADP/Ca2+ pathway may be a novel target for the treatment of autoimmune disorders (Cordiglieri et al., 2010).
Contact

contact information
- telephone: +49 551 3961141
- e-mail address: dmitri.lodygin(at)med.uni-goettingen.de
Relevant Publications
Lodygin D+, Hermann M+, Schweingruber N, Flügel-Koch C, Watanabe T, Schlosser C, Merlini A, Körner H, Chang H-F, Fischer HJ, Reichardt HM, Zagrebelsky M, Mollenhauer B, Frahm J, Stadelmann C, Kügler S, Fitzner D, Haberl M, Odoardi F* & Flügel A*+equal contribution, * co-senior authors
β-Synuclein reactive T cells induce autoimmune CNS grey matter degeneration.
Nature, 566 (2019): 503-508. PMID 30787438.
Diercks BP, Werner R, Weidemüller P, Czarniak F, Hernandez L, Lehmann C, Rosche A, Krüger A, Kaufmann U, Vaeth M, Failla AV, Zobiak B, Kandil FI, Schetelig D, Ruthenbeck A, Meier C, Lodygin D, Flügel A, Ren D, Wolf IMA, Feske S, Guse AH.
ORAI1, STIM1/2, and RYR1 shape subsecond Ca2+ microdomains upon T cell activation.
Sci Signal, 11 (2018): pii: eaat0358. doi: 10.1126/scisignal.aat0358.
Lodygin D, Flügel A.
Intravital real-time analysis of T-cell activation in health and disease.
Cell Calcium, 64 (2017): 118-129.
Schläger C, Körner H, Krueger M, Vidoli S, Haberl M, Mielke D, Brylla E, Issekutz T, Cabañas C, Nelson PJ, Ziemssen T, Rohde V, Bechmann I, Lodygin D, Odoardi F, Flügel A.
Effector T-cell trafficking between the leptomeninges and the cerebrospinal fluid.
Nature 530 (2016): 349-353.
Wolf IM, Diercks BP, Gattkowski E, Czarniak F, Kempski J, Werner R, Schetelig D, Mittrücker HW, Schumacher V, von Osten M, Lodygin D, Flügel A, Fliegert R, Guse AH.
Frontrunners of T cell activation: Initial, localized Ca2+ signals mediated by NAADP and the type 1 ryanodine receptor.
Sci Signal 8 (2015): ra102. doi: 10.1126/scisignal.aab0863.
Lodygin D, Odoardi F, Schläger C, Körner H, Kitz A, Nosov M, van den Brandt J, Reichardt HM, Haberl M, Flügel A.
A combination of fluorescent NFAT and H2B sensors uncovers dynamics of T cell activation in real time during CNS autoimmunity.
Nat Med 19 (2013): 784-790.